Abstract

ATP-competitive BCR::ABL1 inhibitors are effective as frontline treatment for many Chronic Phase (CP) CML patients. However, a significant minority of patients experience treatment failure with currently available drugs, due to resistance or toxicities. Asciminib, a first-in-class drug that inhibits BCR::ABL1 by binding to its myristoyl site, is efficacious in patients who have failed 2 or more lines of treatment (Rea et al, Blood 2021). The Australasian Leukaemia Lymphoma Group (ALLG) CML13 ASCEND-CML trial aims to assess its tolerability and efficacy in the frontline setting.
ALLG CML13 is a prospective open-label phase II study, enrolling 100 patients over 15 Australian and New Zealand sites. Patients commence treatment with asciminib 40mg twice a day (BID), and are assessed thereafter according to optimal 2020 ELN targets (BCR::ABL1 ≤10%, ≤1% & ≤0.1% at 3, 6, 12 months respectively); and ≤0.01% at 18 months. Patients with treatment failure (BCR::ABL1 >10% at 3 or 6 months; BCR::ABL1 >1% at 12 or 18 months) continue asciminib and add either imatinib, dasatinib or nilotinib, according to physician preference. Patients who have not failed, but have not achieved optimal response at 6, 12, or 18 months, have their asciminib dose doubled to 80mg BID. Co-primary end points are achievement of early molecular response (EMR, BCR::ABL1 ≤10% at 3 months) and major molecular response (BCR::ABL1 ≤0.1%) by 12 months. We report a protocol-specified interim analysis of EMR that allows for reporting of repeated confidence intervals. This trial received funding support from Novartis.
By data cut-off at July 15, 2022, the trial remains open to recruitment with 79/100 patients registered, with a median follow-up of 10 (0-19) months. The most commonly reported grade 3/4 adverse events thus far were neutropenia, and thrombocytopenia (n=5 and 4, 5.5% and 5.2%, respectively); as well as increased lipase and amylase without clinical pancreatitis (n=6 & 2; 7.8% & 2.6%, respectively). Grade 3 increased AST/ALT, anaemia, back pain, abdominal pain and infection were each reported once. Nine patients have discontinued asciminib: 1 with persistent grade 4 cytopenia requiring HSCT, 4 with recurrent asymptomatic lipase elevations (including 1 patient who registered but had not started therapy), 1 withdrawn consent and 1 lost to follow-up. Two patients had resistance: 1 had confirmed loss of MMR at 9 months after reaching a nadir of 0.067% at 6 months, without evidence of a kinase domain or myristoyl site mutation; 1 transformed to lymphoid blast crisis at 6 months with myristoyl site mutations A337T (40%), A337V (10%) and P465S (10%), having previously achieved BCR::ABL1 of 0.37% at 3 months.
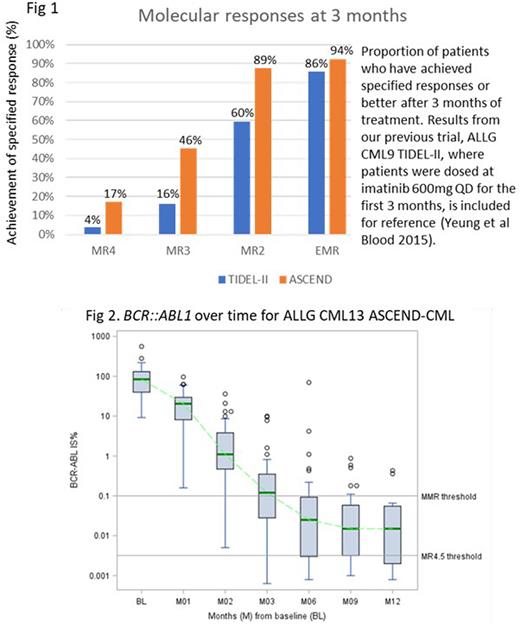
The molecular analysis for EMR includes all registered patients with at least 3 months of follow-up (n=63); 1 patient missed this assessment, and 3/63 patients had withdrawn by this timepoint (all 4 scored as non-responders). EMR was achieved by 59/63 (93.7%; 99.1% CI 81.1-98.9%). By 3 months, 11 patients have already achieved MR4 or better; 29/63 patients achieved MMR (Fig 1 & 2). Three patients had asciminib dose escalation on protocol: 1 patient with BCR::ABL1 of 1.1% at 6 months, and 2 with BCR::ABL1 of 0.34% and 0.43% at 12 months; subsequent molecular results are pending.
Trial interim analysis suggest asciminib to have favorable tolerability and efficacy as frontline treatment for CP-CML. The EMR rate is particularly encouraging, and is likely to translate to improved long term outcomes, with higher achievement of deep molecular responses and potentially treatment-free survival. Equally encouraging is the safety profile and early discontinuation rate. Cytopenia and lipase elevations were the most common adverse events, and appear to be ABL1 inhibitor class effects, though clinically relevant pancreatitis has not occurred.
Disclosures
Yeung:Amgen: Honoraria; BMS: Honoraria, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria. Shanmuganathan:Amgen: Other: Meeting sponsorship; Novartis: Honoraria. Reynolds:Novartis: Current equity holder in publicly-traded company; Abbvie: Research Funding; Alcon: Current equity holder in publicly-traded company. Branford:Cepheid: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Qiagen: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Yong:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Research Funding; Celgene: Honoraria. Shortt:Ostuka: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astex: Research Funding. Viiala:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Forsyth:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other. Browett:BeiGene: Research Funding; Abbvie: Honoraria; Arrowhead: Honoraria; Roche: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Eysa Pharma: Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria. Grove:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: chairperson at local Abbvie Education Event for which my institution received hororaria., Speakers Bureau; Australian advisory boards as below: Consultancy; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees. Grigg:Novartis: Membership on an entity's Board of Directors or advisory committees. Hughes:BMS: Consultancy, Research Funding; Enliven: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.
OffLabel Disclosure:
Asciminib is only currently registered for CML in the 3rd line setting, and is not licensed for use in newly diagnosed patients.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal